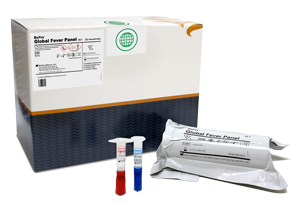
BioFire® Global Fever Panel
BioFire® Global Fever Panel
1 Clinical Test. 6 Targets. All in under an Hour.
With just one test, bacterial, viral and protozoan targets can be identified in whole blood in under an hour with only 2 minutes of hands-on time using the BioFire FilmArray Systems.
For In Vitro Diagnostic Use

BioFire Global Fever Panel – IVD
|
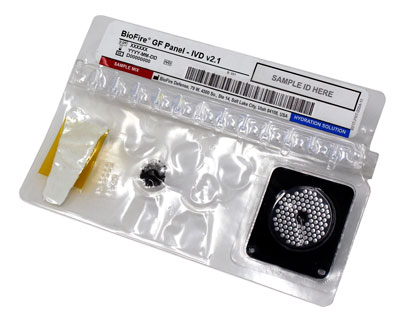 |
|
||||||||||||||||||||||||||||
Software |
||
| BioFire Global Fever Panel – IVD Software Pouch Module for v2.1 (2.1.0.10B) | ||
| BioFire Global Fever Panel ERM Software Download Form (2.1.0.35) |
Technical Notes
|
SDS |
| BioFire Global Fever Panel – IVD SDS Sheet
BIOFIRE SHIELD Global Fever Panel – IVD External Control Kit NHPS |
Reporting |
| Please report adverse events associated with the use of the BioFire Global Fever Panel here. |